Get On-Demand Physician Insights
Book Your Demo Now

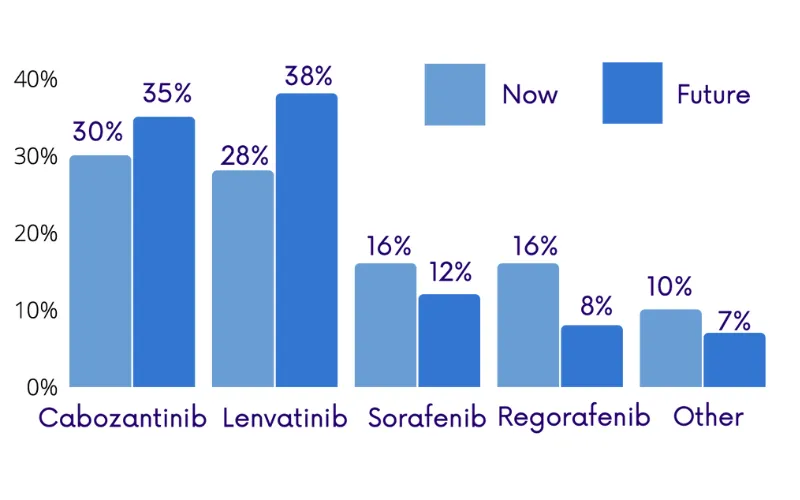



Cabozantinib was approved for HCC 2Ltreatment late last year, it is interesting to see its anticipated use increasein 3L. ‘Other’ treatments are anticipated to be used more often in 3L.
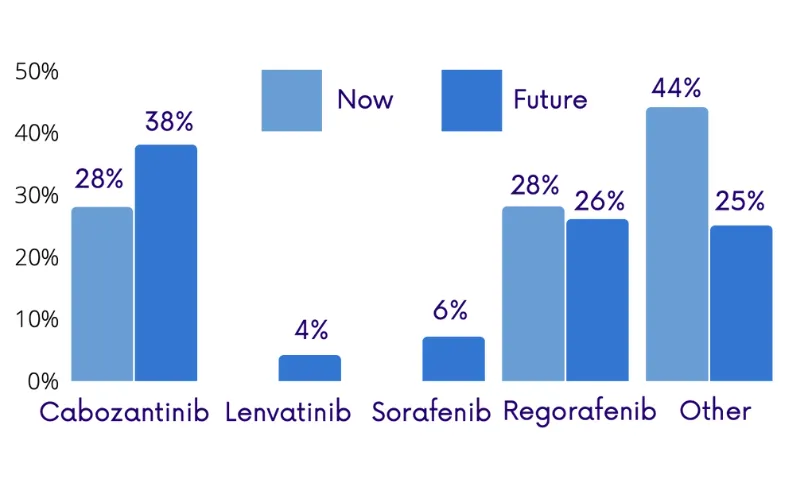
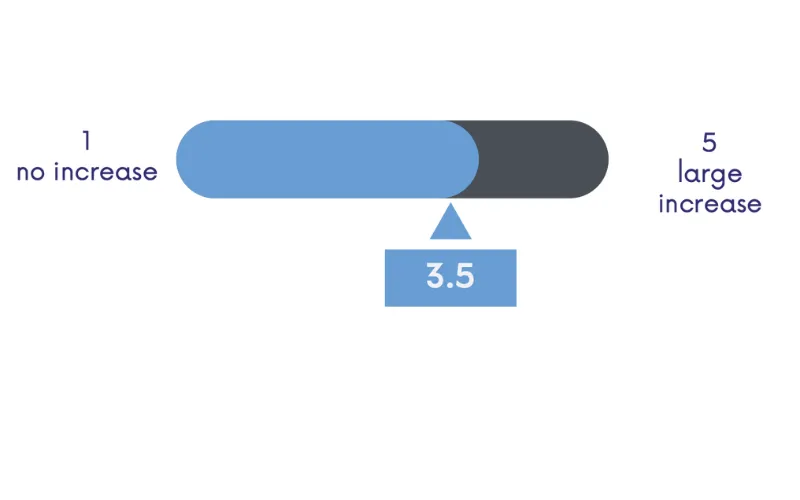

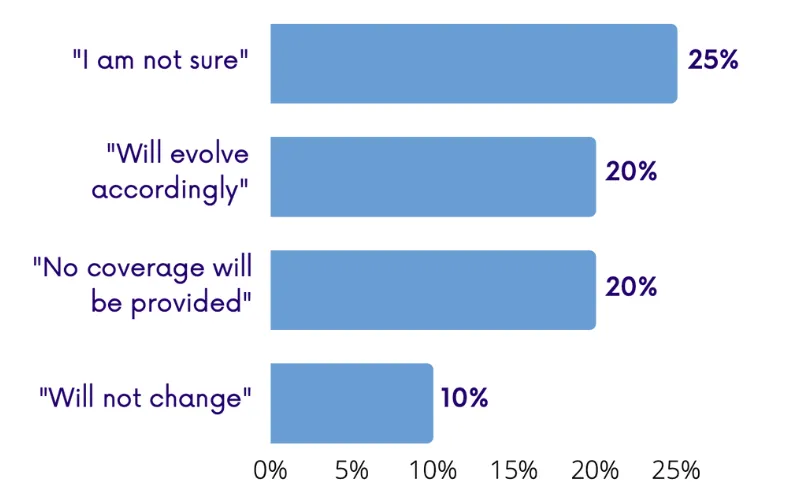
Public health plan coverage for 3L HCC treatment is uneven across provinces, and physicians struck a nihilistic tone when asked if reimbursement policy will improve in their province in the coming months. This finding, along with the expected increase in demand for 3L treatment, suggests that advocacy may be necessary to improve treatment access for 3L HCC patients.
Leave a Comment